Reliable and applicable information circulation system in clinical trials and healthcare settings.

This page shows the "Reliable and applicable information circulation system in clinical trials and healthcare settings." exploried and tested by CMIC Co., Ltd., in the 2023 "Use Case Demonstration Project of the Trusted Web".
Please click on the links at the bottom of this page to learn more about the details of the demonstration.
Current issues and What Trusted Web Solves
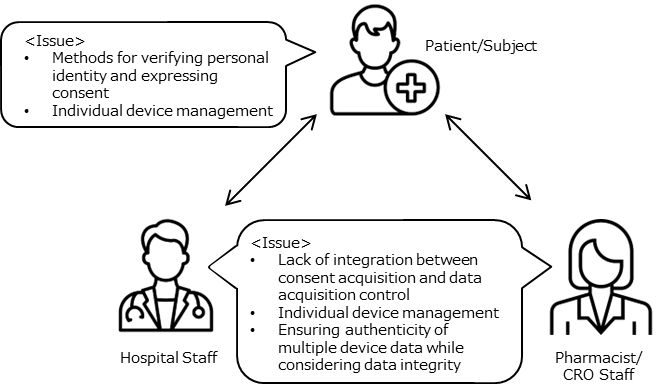
Current Issues (Pain Points)
1. Obtaining Personal Identification and Consent (eConsent)
- In current online medical consultations and remote participation in clinical trials, eConsent is used to verify the identity of the individual by displaying identification documents such as a driver's license and the person's face on a web screen. However, it is difficult to argue that this method is appropriate.
- In face-to-face environments, as opposed to remote environments mentioned above, verification of the individual and obtaining consent for various scenarios such as medical treatment, hospitalization, surgery, and participation in clinical trials are done through signatures on paper documents. However, due to the nature of paper media, seamless integration and coordination with other actions are not possible.
2. Utilizing PHR (wearable devices, etc.)
- In general medical care and clinical trials (DCT), there are difficulties in utilizing data from patients (subjects) on wearable devices, among other reasons. One of the reasons is the lack of a trusted environment and control mechanism, where data can be provided to only those individuals with whom the individual has consented.
- Regarding the above, implementing individual technologies for each wearable device would increase operational costs and impose burdens on users (patients, subjects, and on-site staff), making it impractical.
- From the perspective of data integrity, it is necessary to consider security, personal authentication, and other factors starting from the wearable device as the source of data generation. However, there is insufficient technical verification and investigation regarding the practicality of utilizing these aspects in real clinical settings and clinical trials.
What Trusted Web Will Solve
1. Obtaining Personal Identification and Consent (eConsent)
- Creating personas, implementing DID, and building a trusted relationship with hospital staff (as well as pharmaceutical company/CRO staff) through smartphone applications.
- By pairing with hospital staff (as well as pharmaceutical company/CRO staff), the process of identity verification and consent acquisition is considered complete. This is achieved by displaying an eDocument on the medical institution's device, which includes an eSignature linked to personal information through a DID, thereby visualizing the consent acquisition process (= eConsent).
2. Utilizing PHR (wearable devices, etc.)
- During the pairing process, using DID mapping, multiple wearable devices that are identified as being owned by the same entity are seamlessly paired with the smartphone, which serves as a hub and implements a DID. After pairing, each wearable device can share data within the scope of the consent given in step 1 (data is encrypted and can only be accessed by paired users). Additional coding is done to specify the range of data sharing and provide consent control, in accordance with general medical care or the clinical trial protocol.
- On the smartphone application from step 1, it is also possible to revoke consent (terminate the pairing), and data sharing is halted after consent revocation.
The above steps 1 and 2 are seamlessly implemented, using the initial pairing as the touchpoint.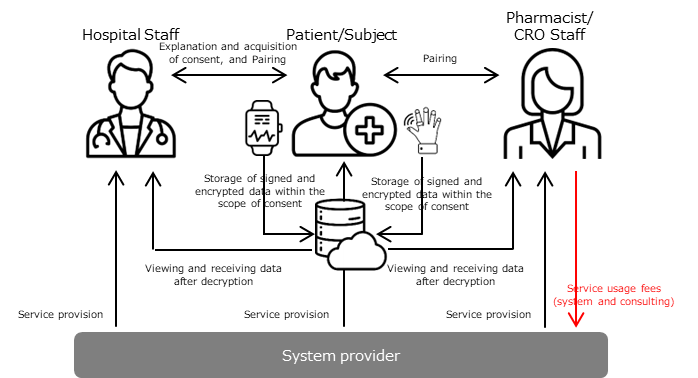
Data to be verified

Consensus building and trace
- By expressing consent for data sharing with third parties, such as physicians, as a touchpoint, a trust relationship (pairing) is established. This is seamlessly achieved through DID mapping implemented on edge devices, allowing data from multiple devices to be shared within the scope of consent. The duration and scope of data sharing are controlled by the data subject, and only stakeholders who have established a trust relationship can perform signature verification on the data.
Business validation (including UX/UI) Development of governance and community activities
- We conducted research on wearable devices compatible with the prototype system of this project and carried out the definition of wearable device requirements taking into account data integrity considerations.
- We organized the governance, rules, business feasibility, and the necessity of data standardization for the utilization of wearable devices such as PHRs in daily medical care and clinical trials through interviews with device manufacturers, pharmaceutical companies, medical institutions, and the PHR service industry association.
- We conducted implementation tests using the prototype system of this project and conducted research on UI/UX.
Achievements
Based on the definition of wearable device requirements, we conducted market research and created the result as an output called "Wearable Device List". Primarily, wearable devices that fall into the following patterns have been introduced to the market, and this project is capable of accommodating each of them.
- Devices that can implement DID (Decentralized Identifier) on the device itself, taking into consideration data integrity.
- Devices that can retrieve data from cloud servers or other sources through APIs (encryption possible using the DID of a smartphone, for example).
Based on the results of the validation tests and stakeholder interviews, the following achievements have been confirmed:
- Based on the clinical trials of DCT (Decentralized Clinical Trial) packages, there is a significant demand for them in the future. They can potentially address existing challenges such as building trust relationships during data provision and operational costs.
- They provide a user interface and user experience (UI/UX) that is tailored to the clinical trial setting, offering easier usability compared to existing systems and ensuring a certain level of usefulness.
- They provide a user interface and user experience (UI/UX) that is tailored to the clinical trial setting, offering easier usability compared to existing systems and ensuring a certain level of usefulness.
- However, there are several challenges that need to be addressed. These include the stakeholders' understanding of the Trusted Web concept, accuracy management and standardization of wearable devices, data format standardization, and the need for guidance and guidelines. It is essential for the industry as a whole to work on understanding the concept of Trusted Web, ensuring data quality and standards, and establishing operational and monitoring systems.
Documents
To communicate with Trusted Web stakeholders,
please use the contact form.