Information distribution system with high reliability and applicability in clinical trials and medical sites(CMIC Co. Ltd.)
This page shows the "Information distribution system with high reliability and applicability in clinical trials and medical sites" exploried and tested by CMIC Co. Ltd., in the 2022 "Use Case Demonstration Project of the Trusted Web".
Please click on the links at the bottom of this page to learn more about the details of the demonstration.
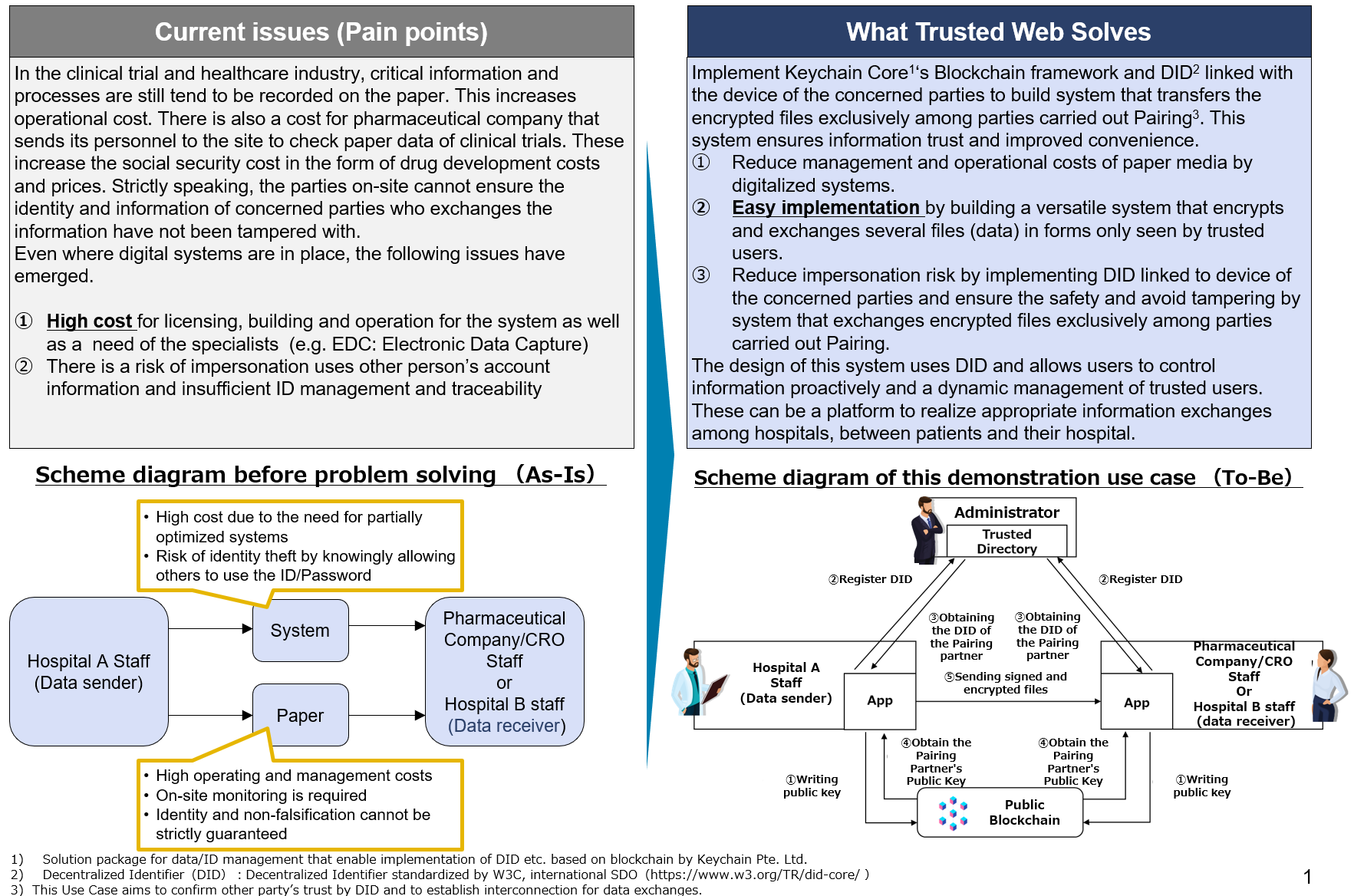
Current issues and What Trusted Web Solves
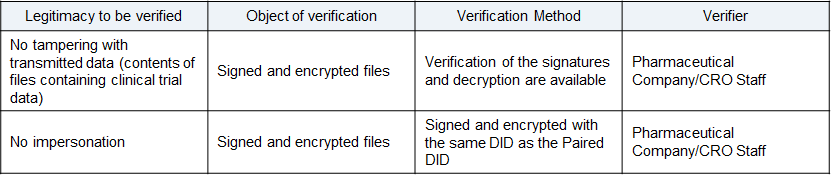
Data to verify
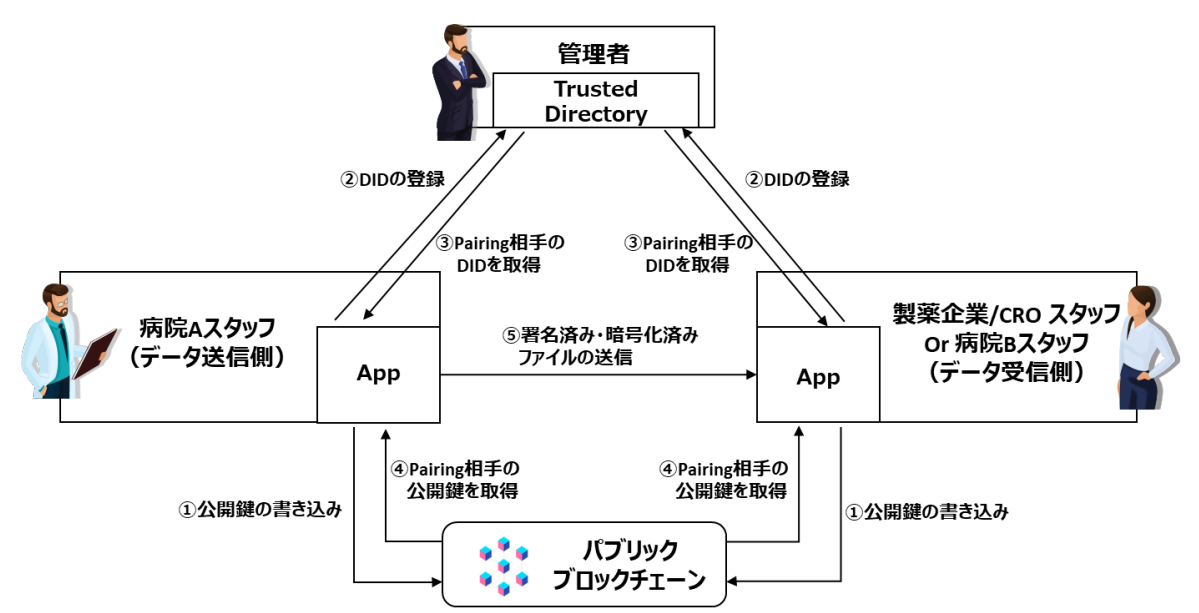
- The sending party signs and encrypts the sending data (includes clinical test data) to secure the data has not been tampered with.
- The received encrypted data can be verified by confirming that the sending party’s DID that performed the Pairing has encrypted the data.
Achievements
During the demonstration period, we conducted a field test of the prototype system between the actual medical institution network and our network with Souseikai Global Clinical Research Center (Souseikai). In addition, we conducted interviews with internal and external experts and stakeholders. The results, suggestions, and comments obtained from these field tests and interviews are as follows.
- (Results) The field test was completed without any problems, and the expected behavior and effects were confirmed. (Comments/Implications) Souseikai's reaction was that they would like to introduce the system to clinical studies and epidemiological studies conducted by medical institutions and academia, which require low-cost planning and implementation and currently do not guarantee data integrity. The possibility of implementing a low-cost model for trials and studies that require a large amount of money for implementation was suggested by simplifying the content of work within the process without making major changes to the existing process itself.
- (Comment) We feel that the architecture of the prototype system and the benefits obtained from it will be an example of what the future should look like. On the other hand, we feel that it is over-engineered compared to the concept of data integrity currently required in the medical field and the clinical trial. Therefore, we feel that activities to disseminate the architecture of this prototype system, and the technical requirements required in the Trusted Web white paper to society as a whole are necessary.
Documents
To communicate with Trusted Web stakeholders,
please use the contact form.